This I/GCSE Chemistry blog post will look into how to prepare different ways of carbon dioxide reactions.
Preparation of Carbon Dioxide
You can collect gases in a test tube, as the gas you are collecting displaces the air in the test tube. You can do this by:
- Upward delivery : to collect gases that are lighter than air
- Downward delivery: to collect gases denser than air.
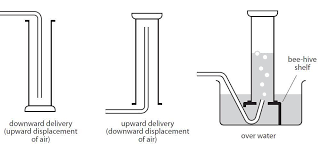
Calcium carbonate are put in the bottom of a flask and dilute hydrochloric acid is added.
This reaction produces calcium carbonate , water and carbon dioxide gas:
- 2HCl + CaCO3 - CaCl2 + H2O + CO2
The carbon dioxide is collected in a gas syringe or using downward delivery.
Thermal decomposition: Heating a metal carbonate also produces CO2, this is an example of thermal decomposition, which is when a substance breaks down into simpler substances when heated.

Copper carbonate is a green powder that will easily decompose to form carbon dioxide and copper oxide.
- CuCO3 - CuO + CO2.
- Carbon dioxide is then collected by downward delivery method
That is all for this post!

References:
https://www.google.com/url?sa=i&url=https%3A%2F%2Fedu.rsc.org%2Fexperiments%2Fthermal-decomposition-of-metal-carbonates%2F450.article&psig=AOvVaw3LfSdh0R9cRaU-7zCYXi0d&ust=1627957329712000&source=images&cd=vfe&ved=0CAsQjRxqFwoTCODC1-WjkfICFQAAAAAdAAAAABAD

