One of the most common questions dealing with the topic of equilibrium would require you to determine the changes brought by the altering of the concentrations of either the products or reactants in a system at equilibrium.
Yes, it can be confusing to many but with certain tricks, you are not far from hitting the bullseye! 👍

🧐 Here’s an example:
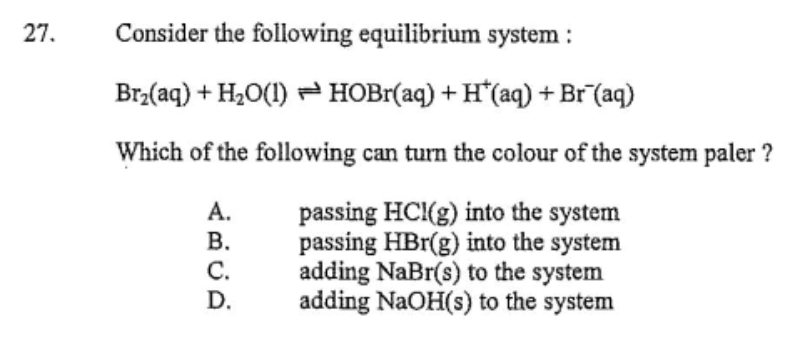
Reminder!!!
Br2 (aq): yellow-brown
Br- (aq): colorless
To tackle this, just ask yourself 2 QUESTIONS 🤔
•Which side should the position of equilibrium shift in order to satisfy the question?
•What changes should be done to the system for the equilibrium position to shift to the required side?
Time to apply this to find the correct answer!💪
First, the equilibrium position should shift to the RIGHT which will increase the concentration of Br- (aq) so the color will get paler.
In addition, the concentration on the reactants side should increase for the equilibrium position to shift to the right.
From the options, only Choice D will cause the equilibrium position to shift to the right. *Adding NaOH (aq) will react with H+ (aq) due to neutralization. Thus, the concentration of H+ (aq) will decrease causing the equilibrium position to shift to the right.
Explanation of the other options
A: Increases H+ (aq)
B: Increases Br- (aq) & H+ (aq)
C: Increases Br- (aq)
*These options will bring about a net backward reaction so the equilibrium position will shift to the left.

Concept Cleared? Hope you enjoy and give us a "Like" 👍
Got Questions Wanna Ask? Whatsapp Us! We Are Here to Help 👂
TUTTEE © All Rights Reserved. Unauthorized Copying is prohibited.

